Every grower will have to do some basic calculations when mixing a nutrient solution. Understanding some of the calculations will help you to apply the correct concentration of a nutrient or determine the concentrations of a combination of nutrients applied. In the previous article Taking Care of Plant Nutrition in Your High Tunnel-Water Hardness and the Removal of Unwanted Ions, we have discussed how to manage hard water, and unwanted high concentrations of sodium, chloride, iron, manganese and sulfur.
Growers have different nutrient solution mixing and application options. Depending on the size of your high tunnel or greenhouse operation and the sophistication level of your nutrient solution application system, you might decide to use a single-bag mix (contains all needed elements), a two-bag mix (Tank A-calcium and iron, and half of potassium nitrate; Tank B-all other elements including phosphates and sulfates), or an individual element mix (individual compound fertilizers). The one-bag mix allows the grower to pick a desired concentration and measure out the exact amount of fertilizer needed. With the two-bag mix, the grower can make-up stock solution tanks at a much higher concentration and then directly inject it at the desired rate into the main water supply line. When making use of individual elements to mix your nutrient solution, a high level of technology is required to inject the exact amounts of fertilizer. All of these actions require some calculations. In this issue, we will discuss some of the basic calculations made.
Water Soluble Fertilizer – The number on the bag indicates the percentage of each nutrient in it e.g. a 5-11-26 fertilizer contains 5% nitrogen, 11% phosphate and 20% potash. Recommendations are usually given in ppm (parts per million) of a specific fertilizer nutrient or in pounds and ounces (weight basis) of a formulation per 100 gallons of water. The manufacturer’s label provides a guaranteed analysis (%) with an indication of what the blend is derived from. It will also provide a summary chart of all elements (ppm) present in the blend. Recommendations on a weight bases do not readily present a specific fertilizer nutrient concentration. However, recommendations based on parts per million (ppm) specify the exact concentration of a specific fertilizer nutrient concentration. Preparation based on ppm take into account that different fertilizers have different nitrogen, phosphorus and potassium concentrations.
Useful Conversions Factors for Calculations
K2O = 83% actual potassium (1 ounce of K2O = 0.83 ounces of K)
P2O5 = 44% actual phosphorus (1 ounce of P2O5 = 0.44 ounces of P)
1 gal. = 3.78 liters
1 oz. = 28.35 grams
1 tablespoon = 3 teaspoons
1 once = 3 tablespoons (dry)
1 ounce = 9 teaspoons (dry)
1 pound = 16 oz. (dry)
Weight Basis Calculation
Scenario 1: A grower receives a recommendation to apply 5-11-26 water-soluble fertilizer to a final concentration of 16 oz per 100 gallons of water.
Question: How much fertilizer should be mixed in a 25-gallon stock tank if an injector with a 1:30 injection rati444o will be used?
Things You Need to Know:
- Recommended fertilizer application rate: 16 oz per 100 gal.
- Fertilizer formulation and analysis: 5-11-26
- Injection ratio: 1:30
- Size of stock tank in gallons: 25 gal.
Step 1: Adjust the rate for the stock tank size using the following equation
Equation: oz per 100 gal. ÷ (100 gal. ÷ stock tank size) = oz fertilizer per stock tank size
Answer: 16 oz per 100 gal. ÷ (100 gal. ÷ 25 gal.) = 4 oz of 5 – 11- 26 per 25 gal. water
Step 2: Adjust the rate for the 1:30 injection ratio (injector will proportion 1 gal. of fertilizer concentrate into every 30 gal. of water).
Equation: oz per stock tank size x injector ratio = oz per stock tank using injector
Answer: 4 oz per 25 gal. x 30 = 120 oz per 25 gal. water using a 1 : 30 injection ratio
Parts per Million Calculation
Scenario 2: A grower receives a recommendation to apply 150 ppm nitrogen using 5-11-26 water-soluble fertilizer.
Question: How much fertilizer should be mixed in a 50-gallon stock tank if an injector with a 1:100 injection ratio will be used?
Things You Need to Know:
- Recommended fertilizer application rate: 150 ppm nitrogen
- Fertilizer formulation and analysis: 5-11-26
- Nitrogen in formulation: 5% (can be presented as decimal fraction (df), 0.05)
- Injection ratio: 1:100
- Size of stock tank: 50 gal.
Step 1: Convert the ppm recommendation to a weight basis using the following equation.
Equation: desired ppm ÷ (df fertilizer nutrient x 75) = oz fertilier per 100 gal. water
Answer: 150 ppm ÷ (0.05 x 75) = 40 oz of 5 – 11 – 26 per 100 gal. water
Where did the 75 come from in the equation?
Step 2: Adjust the rate for the stock tank size using the following equation.
Equation: oz per 100 gal. ÷ (100 gal. ÷ stock tank size) = oz fertilizer per stock tank size
Answer: 40 oz per 100 gal. ÷ (100 gal. ÷ 50 gal.) = 20 oz of 5 – 11- 26 per 50 gal.
Step 3: Adjust the rate for a 1:100 injection ratio.
Equation: oz per 50 gal. x injector ratio = oz per stock tank using injector
Answer: 20 oz per 50 gal. x 100 = 2000 oz per 50 gal. using a 1 : 100 injection ratio
Step 4: Convert ounces to pounds and ounces where, 16 oz = 1 pound (dry).
Answer: (2000 oz ÷ 16 oz) oz per 50 gal. = 125 lbs of 5 – 11 – 26 per 25 gal. for 150 ppm N
How many ppm phosphorus and potassium will be applied with the 150 ppm N from the 5-11-26 fertilizer?
Phosphorus:
oz fertilizer per 100 gal. x 75 x df fertilizer nutrient = ppm actual phosphorus
40 oz × 75 × 0.11 P2O5 = 330 ppm P2O5
For actual Phosphorus: P2O5 is 44% elemental phosphorus
330 ppm P2O5 × 0.44 = 145.2 ppm actual phosphorus
Potassium:
oz fertilizer per 100 gal. x 75 x df fertilizer nutrient = ppm actual potassium
40 oz × 75 × 0.26 K2O = 780 ppm K2O
For actual Potassium: K2O is 83 elemental potassium
780 ppm K2O × 0.83 = 647.4 ppm actual potassium
Finally, the solution will consist of 150 ppm N, 145.2 ppm P, and 647.4 ppm K.
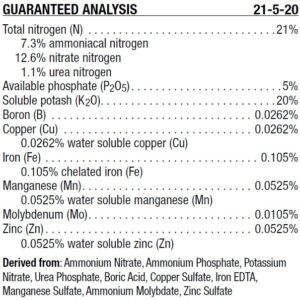
Ammonium and Nitrate Nitrogen Calculation
The following figure illustrates a typical guaranteed analysis of a fertilizer product. From this analysis, we can determine how much ammonium and nitrate nitrogen is in the formulation.
Equation:
% ammonium = (% ammonium + % urea) ÷ % total N) x 100
% nitrate = (% nitrate ÷ % total N) x 100
Answer:
% ammonium = ((7.3 + 1.1) ÷ 21) x 100 = 40
% nitrate = (12.6 ÷ 21) x 100 = 60
The percent ammonium versus nitrate nitrogen in the solution is very useful to know especially if you would like to manage soilless substrate pH, the pH of a recirculating nutrient solution, or if you grow an ammonium sensitive crop.
Potential Acidity and Basicity
The potential acidity and basicity information will also typically appear under ‘Product Properties’ on the ‘Product Analysis’ sheet.
Product 1: The potential acidity (to neutralize acidity produced by fertilizer) of the fertilizer product below is high and indicates that a likely DECREASE in substrate pH will occur. Note that all nitrogen is applied as ammonium nitrogen.
Product 2: The potential basicity (limestone needed to equal the acid neutralizing power of the fertilizer) of this fertilizer product indicates that a likely INCREASE in substrate pH will occur. Note that this formulation contains about 79% nitrate nitrogen.
Alternating fertilizers may help to stabilize substrate pH. It is recommended that the ammonium nitrogen content of a recirculating nutrient solution not exceed 10% of the total nitrogen content.
This is the third article in a 7 part series that look at soil fertility and nutrient solution management for high tunnels. In the next issue, we will concentrate on ‘Fertilizer and Nutrient Solution Mixing Tips’ and a few more calculations’.